
Are you Compliant with the Most Up-to-Date FDA Regulations for Nutritional Labeling?
We’re often asked to provide an overview of dietary supplement labeling requirements for products such as vitamins, herbal supplements, and other functional foods. To ensure your current labels are compliant, here are the requirements for marketing and labeling dietary supplements:
First, What is a Dietary Supplement?
According to the FDA website, the Federal Food, Drug, and Cosmetic Act defines a dietary ingredient as a vitamin; mineral; herb or other botanical; amino acid; or dietary substance used to supplement a diet. Unlike drugs, supplements are not intended to treat, diagnose, prevent, or cure diseases.
It is required that most foods, including dietary supplements, bear nutrition labeling. It’s important to note that although dietary supplements are regulated by the FDA as foods, they’re regulated differently from other food and drugs.
The label of a dietary supplement product is required to be truthful, but also requires the below:

Dietary Supplement Labeling Must Include:
- Name of product
- Net quantity of contents
- Name and address of the manufacturer, packer, or distributor
- Directions for use
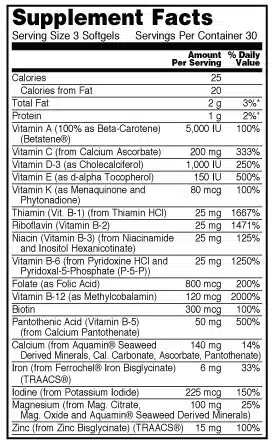
Supplement Facts Panel
A nutrition label for dietary supplements is referred to as a Supplement Facts Panel. The required information includes:
- Serving size
- List of dietary ingredients
- Amount per serving size (by weight)
- Percent of the daily value (%DV)
How to Format a Supplement Facts Panel
The formatting of the Supplement Facts panel is strictly proscribed. For example, the panel must be enclosed in a box. The title must be more significant than all other print, and it, along with headings, must be bolded. So, the type must be easy to read with black lettering and letters that are upper and lower case. If the package is less than 12 square inches in total area, all letters can be uppercased.
While it’s essential to ensure all labeling requirements are met, easy readability and a professional-looking label, are also important and should be top-of-mind. Not only should you clearly communicate to consumers, but you want to attract them to your product as well, which is where AstroNova can help.
Dietary Supplement Labeling Solution
QuickLabel printers provide high-quality, distinctive labels in-house with quick turnaround time, making it effortless to print and update your labels and help your business grow.
Keeping up with the changing regulations can be tiresome and costly. Continually changing regulatory compliance requires supplement manufacturers to adapt their packaging continuously. Therefore, having an in-house label printer, where you can print exactly what you need when you need it, not only provides a competitive edge but also provides peace of mind, not to mention substantial cost savings.
By digitally printing labels in-house, manufacturers can simplify the packaging process and experience greater production flexibility. It’s a wasteful and inefficient practice to buy pre-printed labels in large quantities or find yourself sitting on pre-printed inventory that’s deemed unusable due to a change in label requirements.
With a QuickLabel QL-120X inkjet label printer, the cost per label is affordably low, with no excess label inventory – no wasted labels, no overstocking, and no more outside printing expenses.
Relabeling Supplements
In a global economy, bulk ingredients and finished products are regularly imported and repackaged into individual bottles, jars, rounds, and packs. So, with a QuickLabel in-house label printer, it’s simple to make new supplement labels that comply with domestic labeling regulations.
Enhance product labels with logos, photos, brand images, supplement facts label panels, lot and batch numbers, best-before and expiration dates, color codes, country of origin, manufacturer’s address, and other label content.
How to Submit Your Supplement Label for FDA Review
To submit your dietary supplement labeling to the FDA for a safety review, you must send the information to the Office of Nutritional Products, Labeling, and Dietary Supplements, Center for Food Safety, and Applied Nutrition and Food and Drug Administration. The address is 5100 Paint Branch Parkway, College Park, MD 20740. An original and two copies of the notification must be submitted.
Moreover, a manufacturer is required to submit a notification to the FDA at least 75 days before delivering the supplement for interstate commerce. If a new ingredient is added to a supplement, the manufacturer is required to notify the FDA before it enters the market for consumer use.
Note: If a supplement was developed before October 15, 1994, the FDA does not require that it be reviewed before entering the marketplace.
Looking to Bring Your Labeling In-House?
See for yourself how a QuickLabel in-house label printer can make your supplement labeling more efficient and profitable. Contact us or schedule a free, no-obligation virtual demonstration to speak with one of our specialists.
For more detailed information about labeling dietary supplements, please visit:
http://ods.od.nih.gov/factsheets/DietarySupplements-HealthProfessional/



